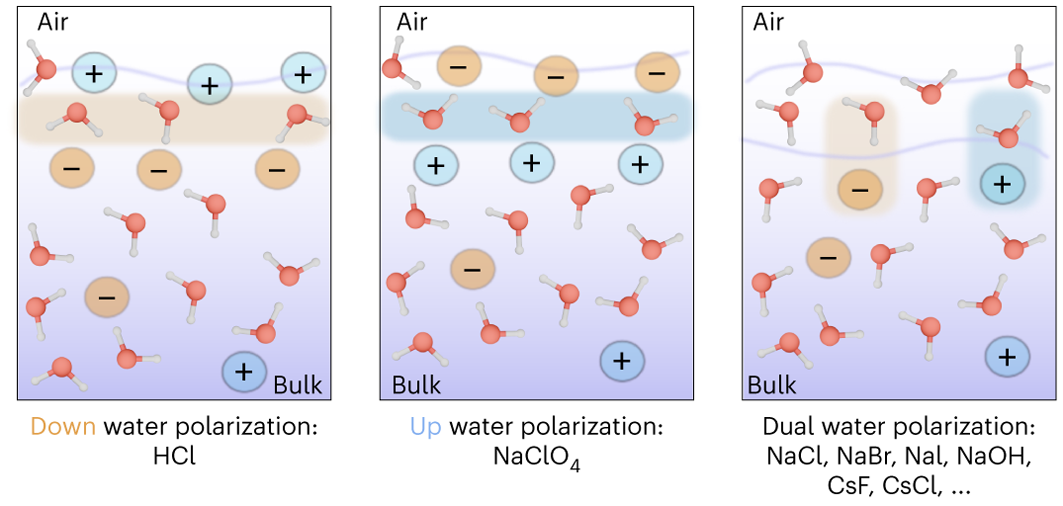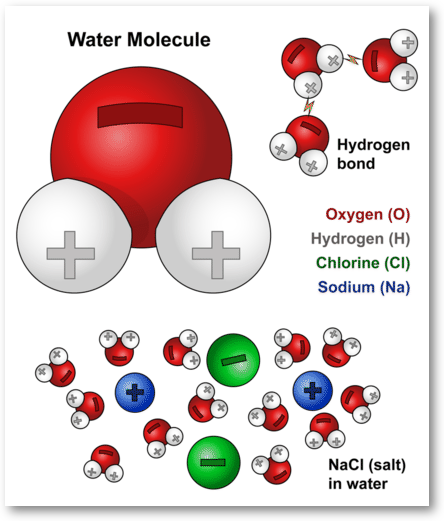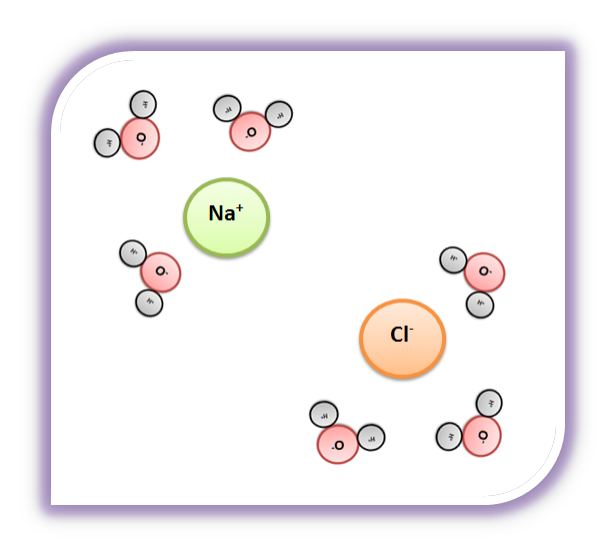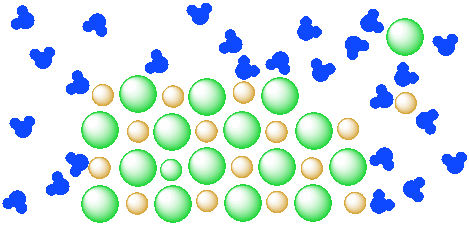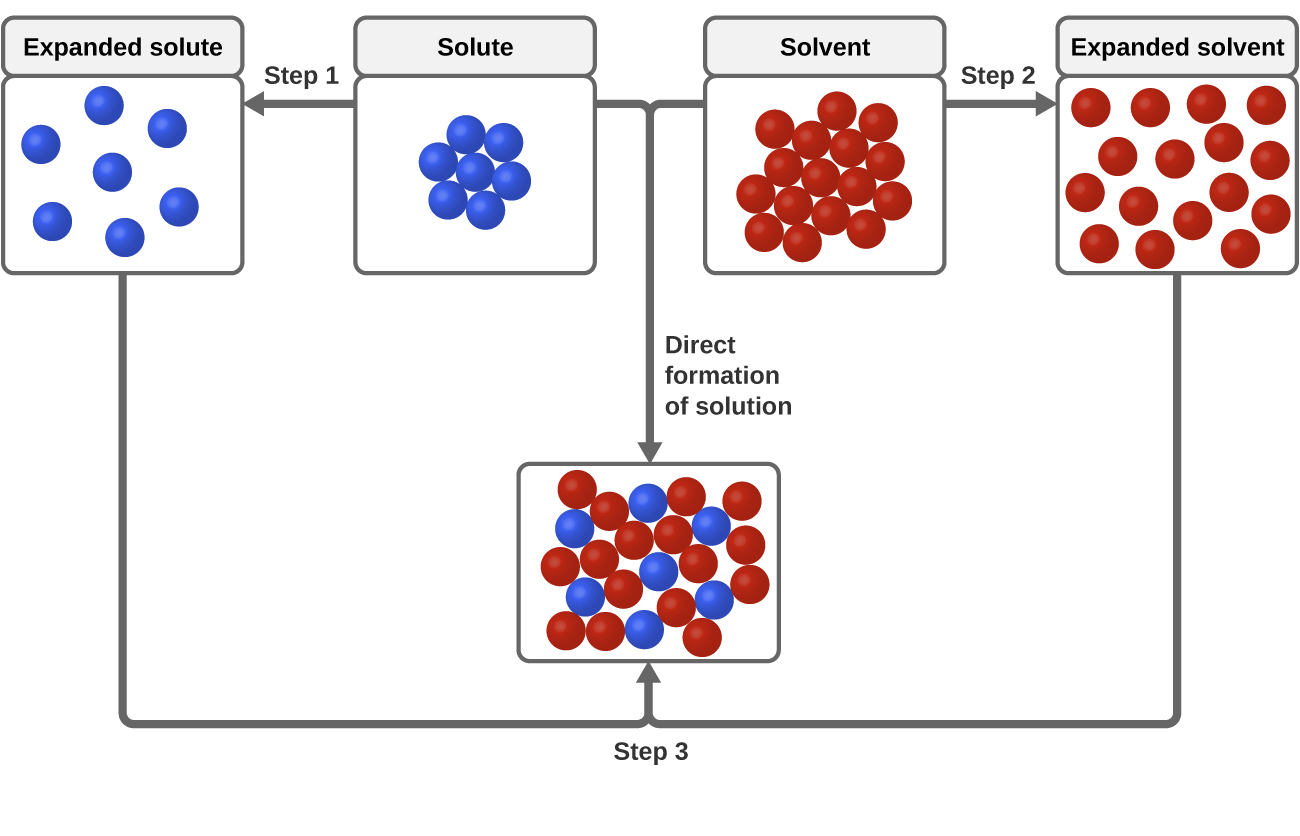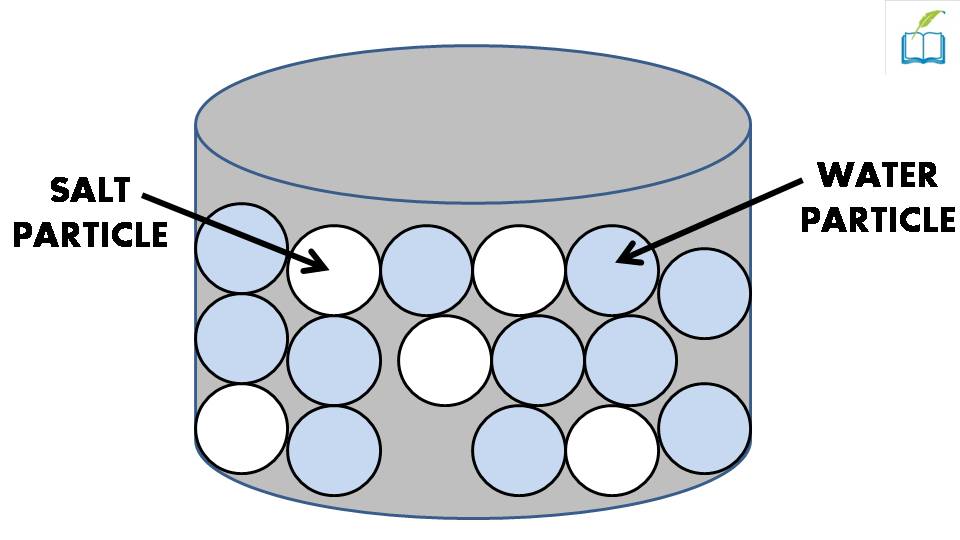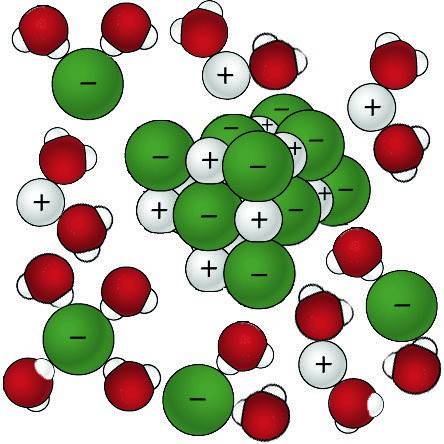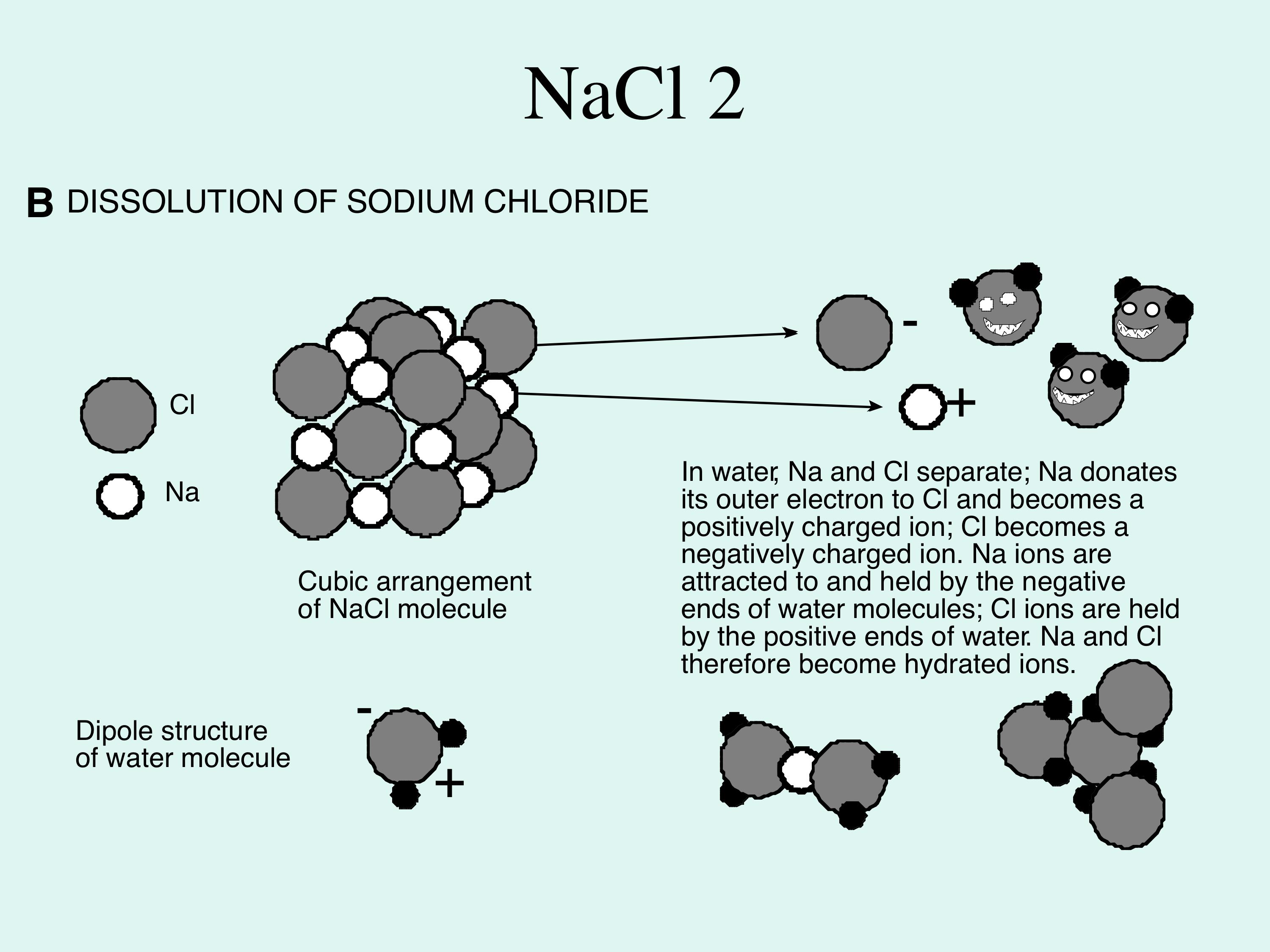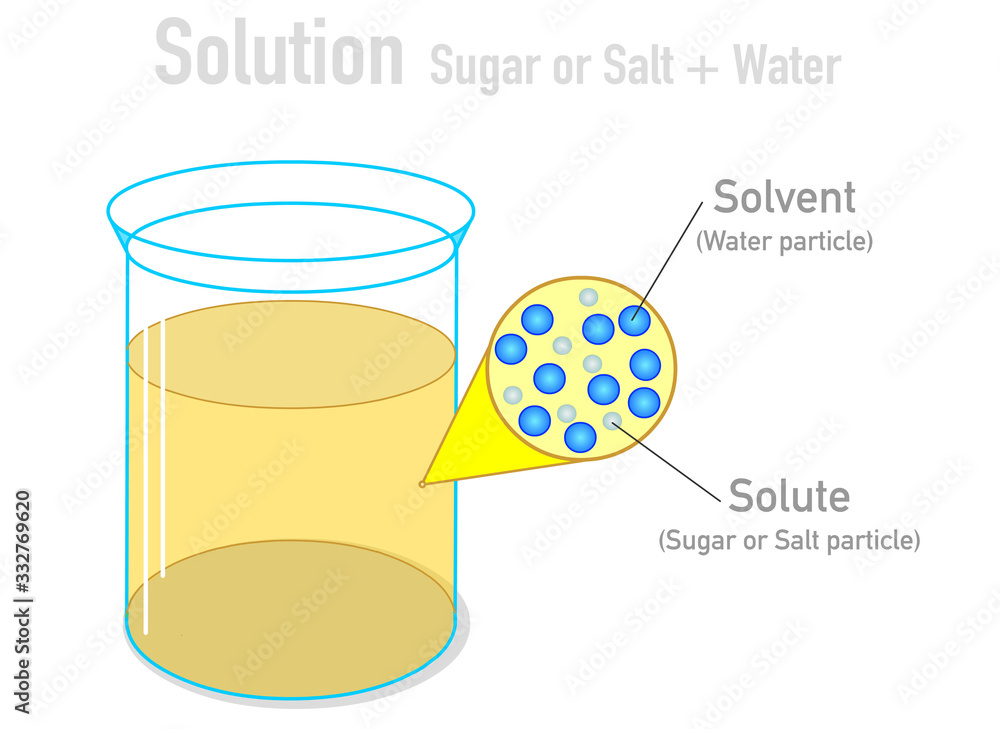
Solvent solute molecules. Salt or sugar water mixture. Homogeneous mixture. Solution under microscope. Solution atoms. Molecular structure. Parts. Liquid mix. White background. Illustration Vector Stock Vector | Adobe Stock
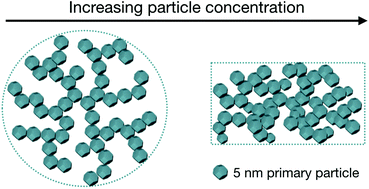
The effect of salt and particle concentration on the dynamic self-assembly of detonation nanodiamonds in water - Nanoscale (RSC Publishing)